Supreme Tips About Do Electrons Actually Carry Energy
Energy Levels Electrons And Covalent Bonding Teach Your Students Vrogue
Electrons
1. What's the Buzz About Electron Energy?
Okay, so you've probably heard about electrons buzzing around atoms, right? Like miniature bees zipping through a microscopic hive. But do these minuscule particles actually carry energy? The simple answer is yes, but the real explanation is a bit more nuanced and, frankly, a lot more interesting. Think of them less like tiny marbles and more like tiny messengers carrying important packages.
The thing is, electrons don't just sit still. They're constantly in motion, orbiting the nucleus of an atom and sometimes even leaping to different energy levels. This movement is where the energy comes in. And it's not just about their speed; it's about their potential to do something. Like, say, power your phone or light up your living room. We're talking about electric potential energy here.
Imagine an electron sitting further away from the nucleus. It's got more potential energy, kind of like a stretched rubber band ready to snap back. When it "snaps back" closer to the nucleus (or moves to a lower energy level), that extra energy has to go somewhere. Often, it's released as a photon — a tiny packet of light. This is how light bulbs work, and it's pretty neat, if you ask me.
So, yes, electrons carry energy. They're the ultimate delivery system for the universe's power grid. But its not like theyre lugging around little batteries. It's more about their position and their ability to change that position, unleashing energy in the process. Think of them as the key players in the world of electricity and energy transformations. Theyre the unsung heroes of almost everything we do.

The Electric Slide
2. From Atom to Application
Let's say you flip a light switch. What happens? Well, it's not magic (although it sometimes feels like it when you stub your toe in the dark). What you're actually doing is setting off a chain reaction of electrons passing energy along. In a wire, for example, electrons are constantly bumping into each other, like tiny billiard balls. Each bump transfers a little bit of energy from one electron to the next. This is what we call electric current.
Think of it like a crowd doing "the wave" at a baseball game. Each person doesn't travel all the way around the stadium, but the wave of energy does. Similarly, electrons don't travel the entire length of a wire, but the energy they carry does, powering your devices. It is a very complicated dance of interactions at the atomic level.
This transfer of energy can also happen through electromagnetic fields. Remember those photons we talked about earlier? When an electron loses energy and releases a photon, that photon can then be absorbed by another electron, giving it more energy. This is how solar panels work. They capture the photons from sunlight, and those photons transfer their energy to electrons in the solar panel material, creating an electric current. Talk about harnessing the power of the sun!
So, electrons are like the ultimate energy carriers, constantly passing the torch (or, more accurately, the photon) to each other. This constant transfer of energy is what makes our modern world possible. Without it, we'd be back to rubbing sticks together for warmth and waiting for the sun to set to know it's bedtime. No thanks!

Energy Levels
3. Understanding Electron Excitement and Relaxation
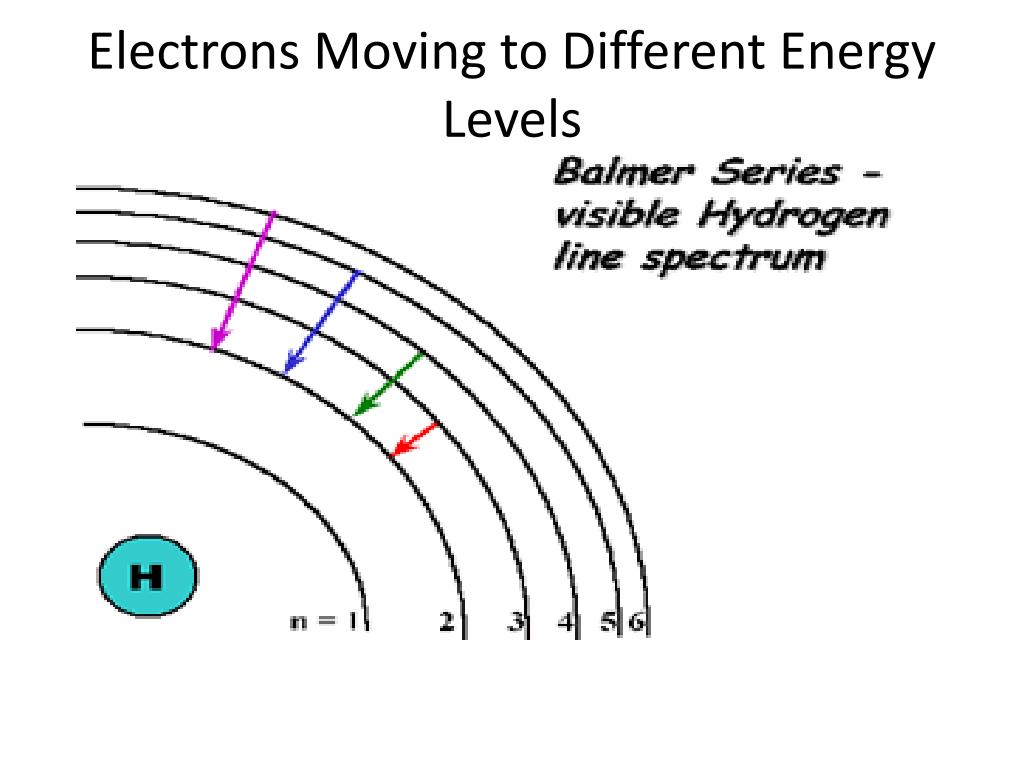
Electrons don't just hang out wherever they please. They exist in specific energy levels around the nucleus of an atom, kind of like floors in a hotel. Some floors (energy levels) are closer to the nucleus (lobby), and some are further away (penthouse). Electrons prefer to stay on the lowest possible floor (lowest energy level) — it's more stable there. However, if you give an electron some extra energy, it can "jump" to a higher floor.
When an electron absorbs energy, whether from a photon or some other source, it gets "excited" and leaps to a higher energy level. But it can't stay there forever. It's like a guest who checks into the penthouse suite but eventually has to check out. After a brief period, the electron will "relax" and fall back down to a lower energy level, releasing the extra energy as a photon or heat.
The color of the light emitted depends on the amount of energy released. This is why different materials glow different colors when heated. For example, a piece of iron heated to a high temperature will glow red, then orange, then yellow, as it emits photons with increasing energy. Each element has its own specific set of energy levels, which means each element emits a unique spectrum of light. This is why we can identify elements by analyzing the light they emit — a technique called spectroscopy. It is like looking at a fingerprint of the elements.
These electron transitions between energy levels are fundamental to many technologies, from lasers to LEDs. Lasers work by stimulating electrons to emit photons of the same wavelength, creating a coherent beam of light. LEDs (light-emitting diodes) use the same principle, but with different materials and a different arrangement, to produce a variety of colors of light. Understanding how electrons carry and release energy at these levels is key to unlocking even more advanced technologies in the future.

Electrons and Electricity
4. The Dynamic Duo of the Modern World
So, we've established that electrons carry energy, and they do it through their motion and their ability to transition between energy levels. But how does this translate into the electricity that powers our homes and businesses? The answer lies in the way electrons behave in materials that conduct electricity, like metals.
In a metal, electrons are not tightly bound to individual atoms. Instead, they're more like a "sea" of electrons, free to move around throughout the material. When you apply a voltage (an electrical "pressure") to a metal wire, these free electrons start to drift in one direction. This flow of electrons is what we call electric current. The more electrons that flow, the stronger the current, and the more energy that is transferred. It's like opening up the floodgates on a river; more water (electrons) flowing means more power (energy) being delivered.
The energy carried by these electrons can then be used to do work, like powering a motor, lighting a bulb, or heating a coil. In each case, the electrons are transferring their energy to the device, causing it to perform its function. Its a wonderfully efficient and versatile way to convert energy from one form to another. You cant see the individual electrons, but you sure can see the result of their collective effort.
Without the free electrons in conductive materials, we wouldn't have electricity. It's a pretty amazing system when you think about it — tiny, almost massless particles carrying the energy that powers our entire civilization. So, next time you flip a light switch or charge your phone, take a moment to appreciate the amazing power of the electron.

Beyond the Basics
5. Exploring the Frontiers of Electron Energy
The study of electrons and their energy is not just about understanding how things work today; it's also about developing new technologies for the future. Scientists are constantly exploring new ways to harness the power of electrons, from developing more efficient solar cells to creating new types of batteries.
One area of research is focused on manipulating electrons at the nanoscale, the level of individual atoms and molecules. By controlling the behavior of electrons at this level, we can create new materials with unprecedented properties. For example, researchers are developing materials that can conduct electricity with almost no resistance, which could revolutionize the way we transmit and store energy. Imagine a world with near-lossless power grids — its within the realm of possibility.
Another exciting area of research is quantum computing, which uses the quantum properties of electrons to perform calculations that are impossible for classical computers. Quantum computers have the potential to solve some of the most challenging problems in science and engineering, from designing new drugs to optimizing energy grids. It is a whole new world of possibilities when we understand how electrons behave at the most fundamental level.
So, the story of electrons and energy is far from over. We're still just scratching the surface of what's possible. As we continue to explore the quantum world and unlock the secrets of electron behavior, we can expect to see even more amazing technologies emerge in the years to come. It's an exciting time to be alive, and the tiny electron is at the center of it all.

Frequently Asked Questions
6. Answering Your Burning Electron Questions
We've covered a lot about electrons and energy, but you might still have some questions. Here are a few of the most common ones:
Q: Do electrons ever run out of energy?
A: Not really. Electrons themselves don't "run out" of energy in the sense that they cease to exist. However, they can lose energy through various processes, such as emitting photons or colliding with other particles. When they lose energy, they simply move to a lower energy state.
Q: If electrons are always moving, why don't we feel them all the time?
A: That's a great question! The electrons are moving within atoms, and atoms are, generally speaking, electrically neutral. So, the positive charge of the nucleus balances out the negative charge of the electrons. Plus, the sheer number of atoms and electrons involved makes it impossible to perceive their individual movements directly.
Q: Are electrons the only particles that carry energy?
A: Nope! Photons, protons, neutrons, and even entire atoms and molecules can carry energy. Energy is a fundamental property of matter and can be transferred between different particles in various ways. However, electrons are particularly important for electrical energy and many chemical reactions.
Q: How can I learn more about electrons and energy?
A: There are tons of great resources online and in libraries. You can start by searching for terms like "atomic physics," "quantum mechanics," or "electricity and magnetism." Many universities also offer free online courses on these topics. Or you could just keep reading awesome articles like this one!