Cant Miss Takeaways Of Tips About Does Anode Or Cathode Go First

Using The Activity Series To Determine Anode And Cathode Complete
Unlocking the Secrets
1. Understanding the Basics of Electrochemical Reactions
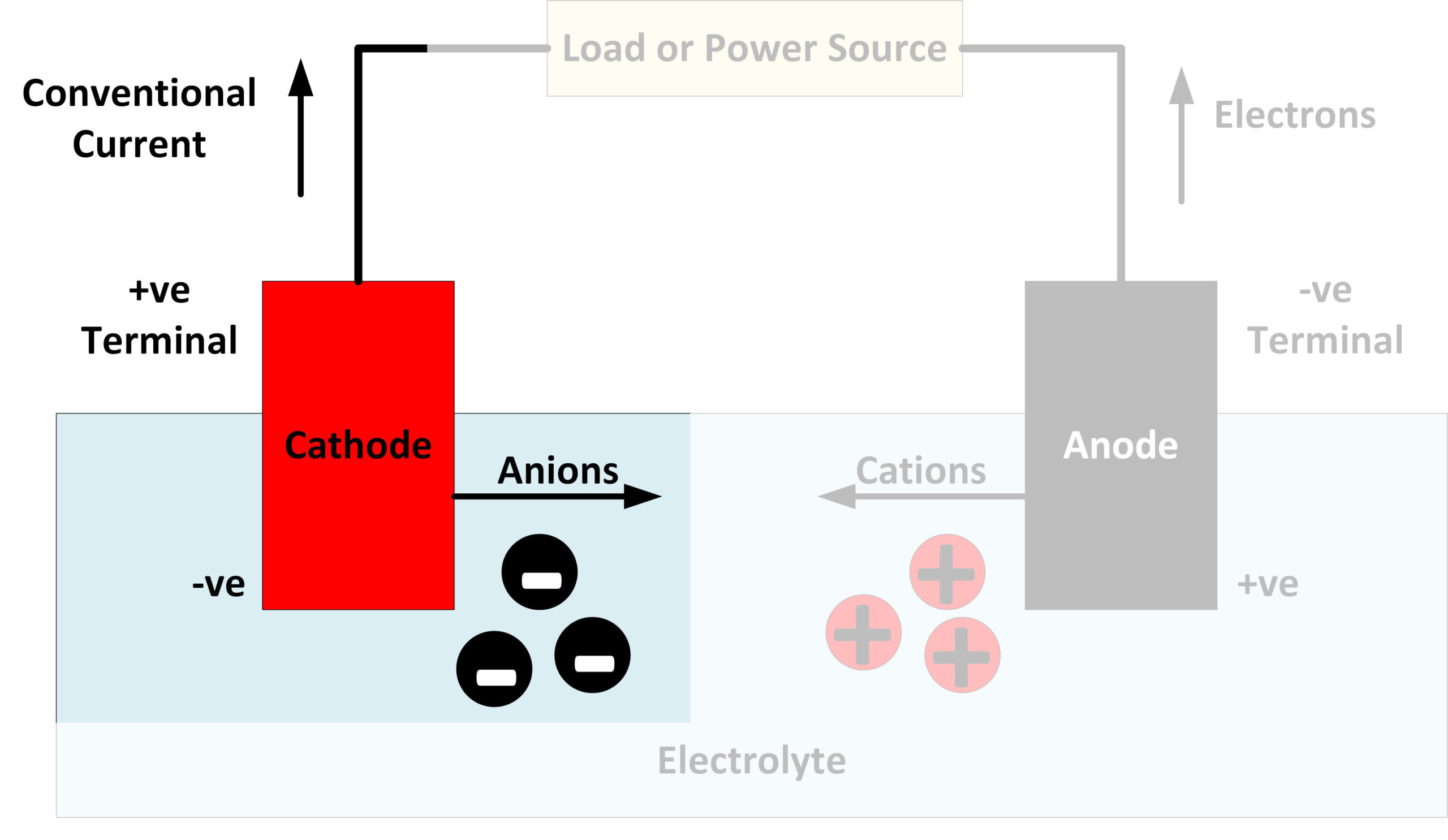
Ever wondered what makes your phone tick, or powers that fancy electric car? It all boils down to electrochemistry, the study of how chemical reactions create electricity (and vice versa). Central to this are two key players: the anode and the cathode. They're like the dynamic duo of electron transfer, but figuring out which one goes "first" can be a bit tricky.
Think of it like this: you've got a battery, and inside, electrons are eager to move. The anode is where they bravely leave the party, giving up their electrons. The cathode, on the other hand, is where these electrons joyfully arrive, ready to participate in another chemical reaction. So, the process starts with the anode releasing electrons.
But hold on, "first" isn't always about a chronological order in the traditional sense. It's more about the direction of electron flow. Its important to remember that the terms anode and cathode are defined by the process happening at each electrode, not necessarily by which one was physically assembled "first" in the manufacturing process. The anode is always where oxidation occurs, and the cathode is always where reduction occurs, regardless of how the cell is constructed.
Essentially, understanding the electron flow helps clear up the confusion. Electrons exit the anode and enter the cathode, driving the entire electrochemical process. So, while there isn't a definitive "goes first" in terms of setting up the experiment, the anode initiates the electron flow.
The Anode
2. Delving Deeper into Oxidation at the Anode
Let's zoom in on the anode. Its job is to undergo oxidation, which basically means it loses electrons. Picture it as a generous donor, happily giving away its electrons to power the circuit. This process is what kickstarts the electrochemical reaction. Without the anode willingly giving up its electrons, the whole system would just sit there doing nothing.
Different materials can serve as anodes, depending on the specific battery or electrochemical cell. Common examples include zinc in alkaline batteries and lithium in lithium-ion batteries. These materials are chosen because they readily give up electrons, making them ideal for the oxidation process.
Interestingly, the anode's material gradually deteriorates as it releases electrons. This is why batteries eventually run out. The anode has given away all its electrons and can't participate in any more oxidation reactions. Its like a runner who has exhausted all their energy during a marathon!
So, in a nutshell, the anode is the unsung hero that initiates the electron flow by sacrificing its electrons. It's the source, the beginning, the electron-releasing champion of the electrochemical world.
The Cathode
3. Exploring Reduction at the Cathode
Now, let's shine a spotlight on the cathode, the anode's partner in crime. While the anode is busy giving away electrons, the cathode is eagerly accepting them. This acceptance of electrons is called reduction. Think of the cathode as the electron's ultimate destination, the place where they finally get to participate in another chemical reaction.
Just like the anode, the cathode can be made of various materials, depending on the electrochemical cell. Common cathode materials include manganese dioxide in alkaline batteries and lithium cobalt oxide in lithium-ion batteries. These materials are chosen because they readily accept electrons, facilitating the reduction process.
Unlike the anode, the cathode doesn't usually deteriorate as it accepts electrons. It acts more as a catalyst, facilitating the reduction reaction without being consumed itself. Think of it as a gracious host, welcoming the electrons and facilitating the reaction without getting directly involved.
Therefore, the cathode is essentially the electron's receiving point, the final destination in the electrochemical journey. Its where the electrons trigger another reaction, continuing the flow of electricity. It's like the end of a conveyor belt, where the product is finally processed and used.
Putting it all Together
4. The Anode-Cathode Connection
Alright, let's connect the dots. The anode gives up electrons (oxidation), and these electrons travel through an external circuit to reach the cathode. The cathode then accepts these electrons (reduction), completing the circuit and driving the overall electrochemical reaction. This whole dance is what makes batteries and other electrochemical devices work.
Imagine a water wheel: the anode is the source of the water (electrons), the wheel is the external circuit, and the cathode is where the water finally flows, turning the wheel and doing work. Without both the source and the destination, the wheel wouldn't turn.
The voltage of the electrochemical cell — the force pushing the electrons through the circuit — is determined by the difference in electrochemical potential between the anode and the cathode. A larger difference means a higher voltage, and therefore more power.
Therefore, the anode and cathode work in perfect harmony, creating a continuous flow of electrons that powers our world. One can't function without the other; they're a true team, working together to create electricity from chemical reactions.

Cathode And Anode Key Differences & Definitions CollegeSearch
Practical Implications and Real-World Examples
5. From Batteries to Fuel Cells and Beyond
So, how does all this anode-cathode talk translate to the real world? Well, think about batteries again. They're a prime example of electrochemical cells in action. The anode and cathode materials determine the battery's voltage, capacity, and lifespan. Different battery types use different materials to optimize performance for specific applications.
Electric vehicles also rely heavily on electrochemical reactions. The battery packs in these cars are composed of numerous individual electrochemical cells, each with an anode and a cathode. The performance and range of an electric vehicle depend on the efficiency and energy density of these cells.
Even fuel cells, which generate electricity from chemical fuels like hydrogen, rely on the anode and cathode. In a fuel cell, hydrogen is oxidized at the anode, and oxygen is reduced at the cathode, producing electricity and water as byproducts.
Essentially, the understanding of anode and cathode behavior is crucial in designing and improving various energy storage and conversion technologies. From small portable devices to large-scale energy systems, the principles of electrochemistry govern their operation.
Frequently Asked Questions (FAQs)
6. Answering Your Burning Anode and Cathode Questions
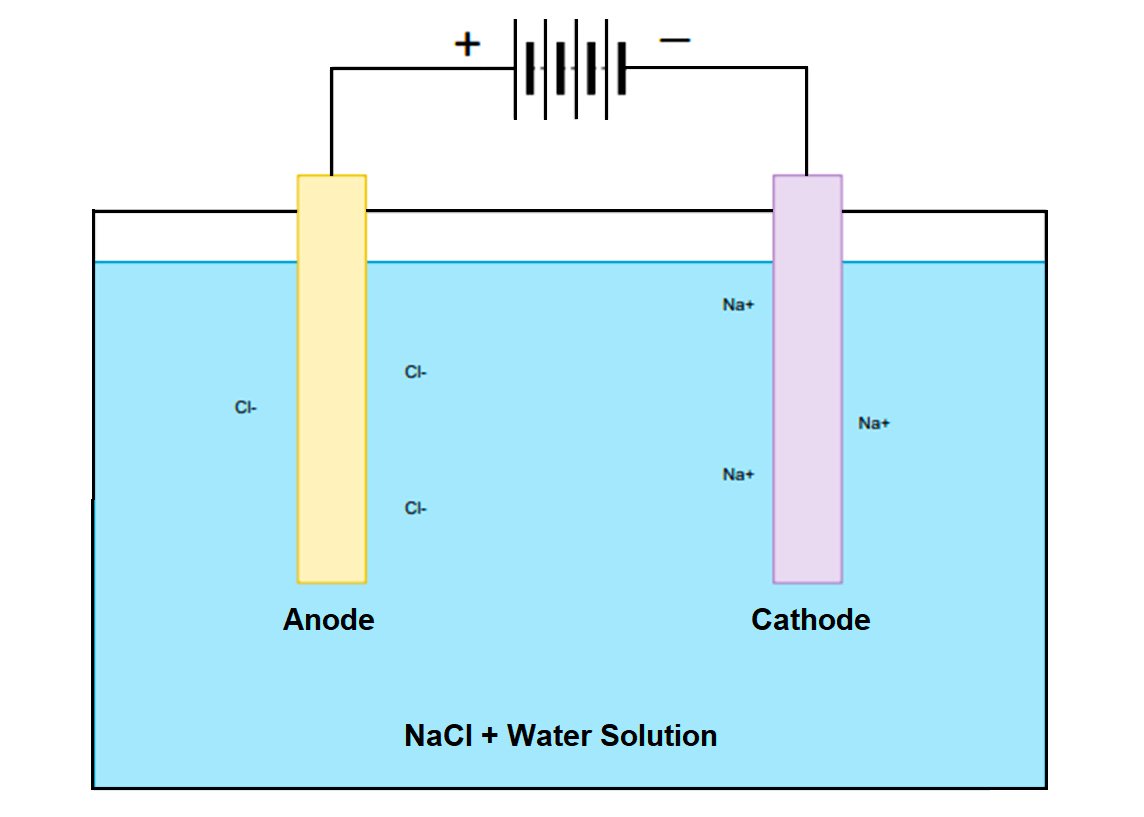
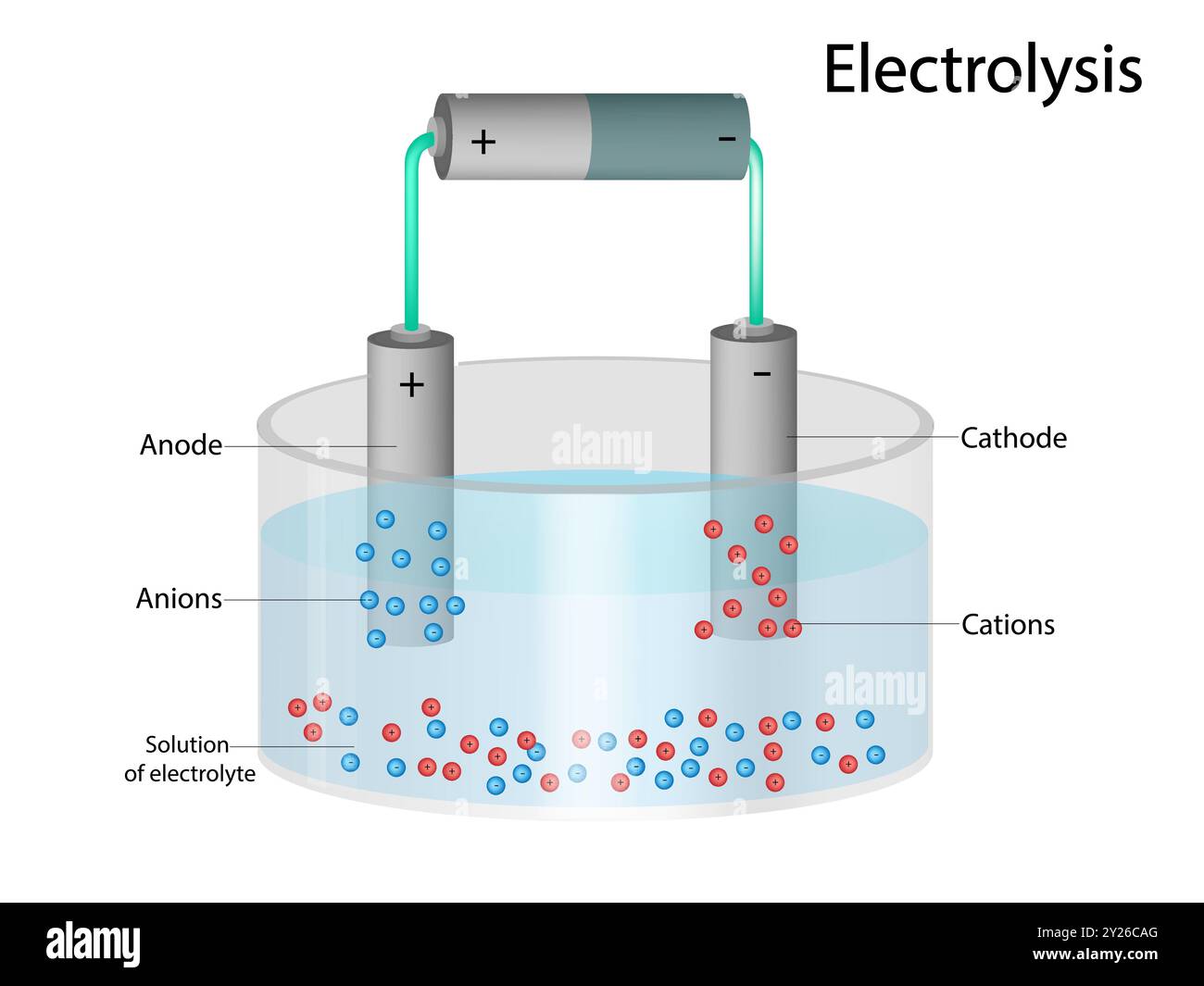
Q: Does the anode always have a negative charge?A: Not necessarily! It depends on whether we're talking about a galvanic cell (like a battery) or an electrolytic cell (where electricity is used to drive a non-spontaneous reaction). In a battery, the anode is negative, but in electrolysis, it's positive. The key is oxidation always happens at the anode, regardless of the charge.
Q: What happens if you reverse the anode and cathode connections?A: In a battery, reversing the connections typically won't work. The device is designed for electrons to flow in one direction only. In some cases, it could even damage the device or the battery itself. In electrolysis, reversing the electrodes will reverse the reaction that is occurring.
Q: Can the same material be used as both an anode and a cathode?A: Technically, yes, in certain specialized electrochemical systems. However, for practical batteries and fuel cells, different materials are usually chosen for their specific oxidation and reduction properties. Efficiency will be very poor.